Abstract
Introduction: The randomized phase 3 ALFA-0701 study demonstrated improved outcomes with the addition of gemtuzumab ozogamicin (GO) to standard 7+3 chemotherapy in patients with de novo acute myeloid leukemia (AML; Castaigne et al, Lancet 2012). 85 of 271 patients in the study received hematopoietic stem cell transplantation (HSCT) at any time during study. Here we describe transplant characteristics and compare survival and veno-occlusive disease (VOD) outcomes in these patients.
Methods: Patients aged 50-70 years with de novo AML who received standard chemotherapy treatment (daunorubicin and cytarabine) with the addition of GO (GO arm) or alone (control arm) and underwent HSCT as part of the open-label, randomized phase 3 ALFA-0701 study were included in this analysis. Patients who experienced complete remission could be considered for allogeneic HSCT according to performance status, age, the existence or not of a related donor, and cytogenetic and molecular risk categories. Allogeneic HSCT was considered for patients in the European LeukemiaNet (ELN) intermediate 2 or adverse risk group. The type of pre-transplant conditioning regimen was left to the discretion of the transplant center. An interval of 2 months between the last dose of GO and HSCT was recommended. Post-transplant survival was defined as the time from HSCT to death due to any cause. Data on VOD events were extensively collected from screening up to the patient's death or the data collection cutoff date (November 1, 2013), whichever occurred first.
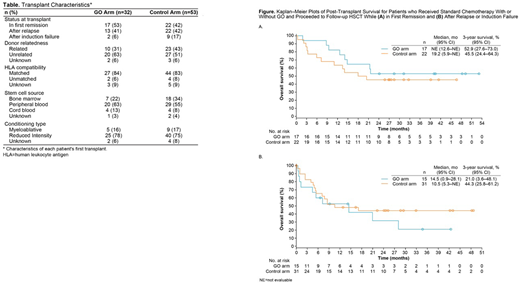
Results: A total of 32 patients in the GO arm and 53 patients in the control arm underwent HSCT (Table). All patients received allogeneic HSCT except for 1 patient in the control arm who received autologous HSCT. In the GO arm, 17 patients received HSCT in first remission, 2 after induction failure, and 13 after relapse. In the control arm, 22 patients received HSCT in first remission, 9 after induction failure, and 22 after relapse. Median (range) age was 60 (51-67) years in the GO arm and 59 (50-69) years in the control arm, and 47% and 45% were male, respectively. The distribution of ELN risk classification was similar for the GO vs control arm, respectively (favorable/intermediate: 63% vs 72%; adverse: 28% vs 25%). Only 1 patient in the control arm who received GO as follow-up therapy received transplant <2 months after the last GO dose.
As of April 30, 2013, 18 (56%) patients in the GO arm and 28 (53%) in the control arm had died: 7 and 16 of these deaths were attributed to disease progression/relapse, and 4 and 7 were attributed to graft versus host disease, respectively. Median post-transplant survival was 21.4 months in the GO arm vs 17.1 months in the control arm, with 3-year survival probability (95% confidence interval [CI]) of 39% (22-57) vs 45% (31-58), respectively (hazard ratio [HR]=0.97). Median post-transplant survival was not yet reached in the GO arm vs 19.2 months in the control arm for patients who received HSCT while in first remission, with 3-year survival probability (95% CI) of 53% (28-73) vs 46% (24-64), respectively (HR=0.71; Figure). In patients who received HSCT after relapse or induction failure, median post-transplant survival was 14.5 vs 10.5 months in the GO vs control arm, with 3-year survival probability (95% CI) of 21% (4-48) vs 44% (26-61), respectively (HR=1.42).
In all, 3 patients in the GO arm and 2 in the control arm (both of whom received GO as follow-up therapy) developed VOD; 3 of these cases (2 in GO arm; 1 in control arm) occurred post-transplant. All but 1 patient fully recovered.
Conclusions: Similar post-transplant outcomes were observed in patients with AML treated with standard chemotherapy with and without GO. The use of GO was not associated with an excess of VOD events after HSCT. The results suggest that the administration of GO as part of induction and consolidation chemotherapy for AML does not induce excess post-transplant mortality and thus does not preclude the use of transplant as consolidation treatment following induction or salvage treatment.
Benner:Pfizer: Employment, Equity Ownership. Vandendries:Pfizer Inc: Employment, Equity Ownership. Gogat:Pfizer Inc: Employment, Equity Ownership. Castaigne:Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal